Cancer Screening in Five Continents (CanScreen5) – a project designed to improve the quality of cancer screening programmes
Eric Lucas, Dr Andre L Carvalho and Dr Partha Basu, Screening Group, International Agency for Research on Cancer, Lyon, France
The Cancer Screening in Five Continents project (CanScreen5) of the International Agency for Research on Cancer (IARC) is designed to uniformly collect, analyse, store and disseminate information on the characteristics and performance of cancer screening programmes in different countries, with the core objective of motivating and supporting the countries to collect and use cancer screening data in a consistent manner on a regular basis using an effective information system. A web-based open access platform will be created to reflect data from the screening programmes across the globe and allow the screening programmes to compare their performance over time and with other similar programmes. The new initiative will impress upon the programme managers the value of monitoring and quality improvement of cancer screening programmes and also support capacity-building in this area.
Cancer screening programmes are complex and resource-intensive but can have huge benefits when implemented in the right manner. Systematic screening of the population at risk for some of the common cancers can significantly reduce the mortality from the disease. However, this requires appropriate planning, adequate financial, human and technical resources and high level of organization of the health services (1). Lack of governmental commitment to provide the requisite sustainable resources can be a serious barrier to successful implementation of cancer screening programmes (2). Countries that do not have adequate resources, infrastructure and health system coordination to implement cancer screening should prioritize early diagnosis of symptomatic individuals linked with prompt and good-quality treatment (3). Currently, population-based screening is recommended only for cancers of breast, cervix, colorectum and oral cavity (4). Following the success of cancer screening in high-resourced countries, many of the countries with limited resources have included population-based cancer screening in their national cancer control plans. Romero et al. reported that already 133 countries included cervical cancer screening and 120 included breast cancer screening in their national plans (5). However, few of them included adequate budget allocation, a comprehensive implementation plan and a strategy for quality assurance.
Many of the countries in Europe have heavily invested in implementing cancer screening programmes over the last few decades. In 2003, the Health Ministers of the European Union (EU) adopted a set of recommendations on cancer screening delineating the key principles of planning, implementing and evaluating quality-assured programmes, and invited all member states to implement breast, cervical and colorectal cancer screening using a population-based approach. The European Guidelines were published to provide evidence-based recommendations to the Member States and also to highlight the necessity of regular monitoring and evaluation (6,7,8). The International Agency for Research on Cancer (IARC) in Lyon, published the reports on the status of implementation of cancer screening programmes in the EU in 2008 (first report) and 2017 (second report) (9,10). These reports described the protocols, level of organization, status of implementation and performance of the screening programmes in the EU region. Similar reports are regularly published by the screening programmes in Australia, Canada and some of the countries in Europe outside the EU. However, the vast majority of countries in low- and medium-resourced settings do not have sufficiently organized screening programmes to report the status of implementation and performance on a regular basis (11). In 2016, the World Health Organisation (WHO) launched the non-communicable diseases (NCD) document repository, which provides access to over 2,900 documents containing NCD targets, policies and guidelines submitted by Member States to WHO, including cancer control guidelines for some countries (12). However, there is a lack of a global database that uniformly gathers and stores information on cancer screening programmes in a standardized manner that could reflect data from the real world and would allow comparisons between countries. The cancer screening in five continents (CanScreen5) project of IARC proposes to build such a global repository reference of accurate information on the cancer screening programmes and their performance worldwide.
Aims and objectives of CanScreen5
CanScreen5 is a global project designed to collect, analyse and disseminate information on cancer screening programmes and activities in different countries with the core objective of motivating and supporting countries to collect and utilize cancer screening data in a consistent manner on a regular basis, utilizing an effective information system. Capacity-building of service providers and programme managers in collecting good quality data for better programme evaluation and quality improvement is a major focus of CanScreen5. The specific objectives are:
- Periodically report the status of implementation of cancer screening programmes at national and sub-national levels in different countries in five continents;
- Evaluate the population-based cancer screening programmes in different countries using standardized process and outcome indicators;
- Share the information with policy-makers, programme administrators, researchers and other stakeholders with an objective to improve the quality of cancer screening programmes;
- Report the use of novel screening tests, screening algorithms and population-based approaches followed by different cancer screening programmes;
- Impart training on monitoring, evaluation and quality assurance to the programme coordinators, data managers and other personnel involved in monitoring and evaluation; and
- Support collaborative research aiming towards the evaluation of efficient and effective approaches to population-based cancer screening.
Methodology
The CanScreen5 project is founded on the IARC experience in preparing the two status reports on the implementation cancer screening in the EU (9,10). The tools and strategies for data collection developed to prepare the reports have been further refined to make them suitable for different resource settings, especially for LMICs. A web-based open access portal is developed with technical support from CPO Piemonte, Italy. All data published in the second EU report on implementation of breast, cervical and colorectal cancer screening from the 28 European Member States are migrated and available on the platform. The synthesized data is presented for a given cancer type (cancer fact sheets), for a selected country (country fact sheets) or through the analysis tools (tables, graphics or map format) (Figure 1). We plan to officially launch the CanScreen5 platform by middle of 2019, initially only the data from the 28 European Member States will be available. After the launch of the platform, we will invite the representatives from the Ministry of Health, programme coordinators and researchers involved in managing and/or supervising cancer screening programmes in different countries to collect and share qualitative and quantitative information on their respective programmes. The data-providers should obtain a mandate from the Ministry or national authorities to share the data. The selected data providers will be trained on the functionality of the CanScreen5 platform, the data collection methods, the standard definition of the performance indicators and their significance in programme quality assurance before they are given password-protected access to the platform to upload their data. Expected information to be collected comprise those on national policies, protocol for screening and diagnosis, programme management, financing, inviting the target population, screening practices, quality assurance planning, including screening registries, etc. A cancer site-specific quantitative data collection questionnaire will be used by the data-providers to collect aggregated data related to the number of invitations sent, number of individuals screened, number further assessed and further assessment results. Once such data are uploaded on the CanScreen5 platform, the inbuilt data analysis software will automatically estimate the process indicators (population coverage, participation rate, compliance to further assessment, etc.) and the outcomes indicators (screening test positivity, detection rate of disease, predictive values of tests, etc.).
The data submitted by the data providers will undergo quality checks before the analysed data is displayed and disseminated through the web platform. The project secretariat at IARC will perform the initial review to check for consistency, completeness, and validity of the aggregated data. The compiled and analysed data will be shared for further validation with the CanScreen5 Scientific Committee for final validation.
The project will be implemented in phases, initially targeting the countries having a reasonable degree of organization of the cancer screening programme and a functioning health information system (HIS). Tailored approaches to data collection will be formulated for the countries not having efficient screening registry or health information system. However, appreciating the challenges that might be encountered in the majority of the countries to collect data of adequate quality and completeness, the Scientific Committee members will decide on the most pragmatic way to collect authentic information from the different countries and the “minimum dataset”’ that needs to be collected from reliable sources for performance evaluation. The countries failing to provide the “minimum dataset” will only have the qualitative information collected and analysed. The CanScreen5 project will collect data from population-based programmes, opportunistic programmes or pilot/demonstration projects, and population surveys and categorize them according to the quality and reliability of source.
A partnership will be developed with major international cancer organizations and foundations involved in supporting and evaluating cancer screening programmes and initiatives.
Discussion
Earlier studies have highlighted the low quality or complete lack of data to evaluate the cancer screening programmes in Latin America and other regions, in spite of the large volume of screening activities in many of them (13). The European experience demonstrated the significant contribution of screening registries to improve the data quality and completeness and better organization of the programmes (14). It is now well-recognized that a robust health information system is extremely critical for the success of a cancer screening programme. The screening programme information system (screening registry) should consist of a minimum dataset, comprising individual information, screening test findings, confirmation/clinical assessment outcomes, referral for treatment, final histopathology diagnosis and stage of cancer. A working group chaired by Dr A Anttila in 2011, recommended the procedures, the data items needed and the coding structures for a systematic individual-level registration of cancer screening programmes (15). The working group also provided a set of key performance indicators that could be relevant for the European screening programmes and defined them. The CanScreen5 project adapted the performance indicators and the methodology of estimating them from these European recommendations but simplified and refined them to be globally relevant. We expect that by encouraging the systematic reporting of the characteristics and the outcomes of the screening programmes, monitored and evaluated using performance indicators against a set of standards, the CanScreen5 project will help continuous quality improvement of the programmes, reduce inequity and harm and promote better utilization of the resources.
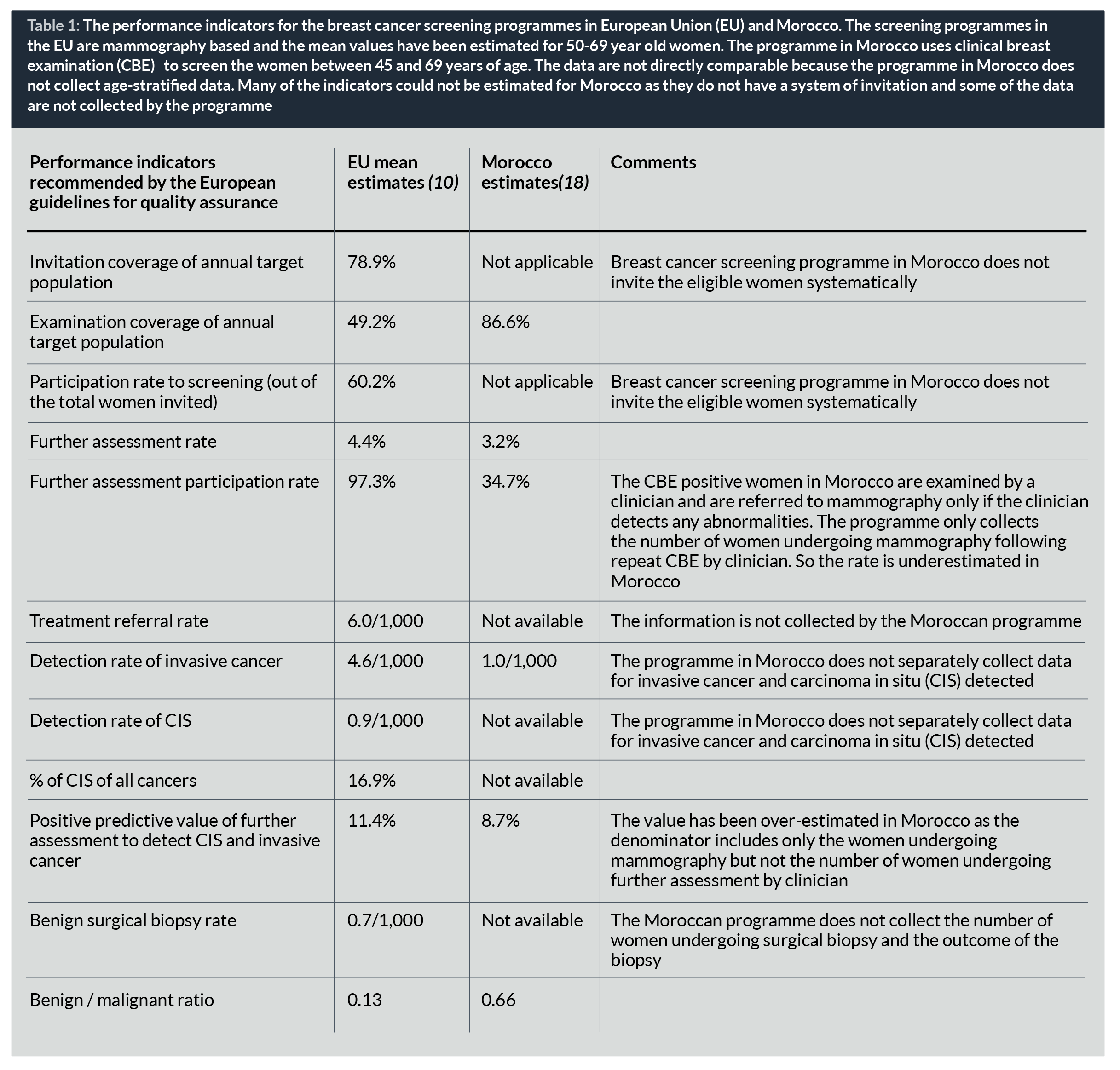
LMICs have many difficulties in implementing organized population-based cancer screening programmes; these hurdles include sociocultural and educational barriers, access to healthcare, lack of trained health professionals, fragmented healthcare systems and financial cost, among others, but also the lack of systematic collection of performance indicators that could be used as the basis for continuous quality improvement of the programme (3,16,17). In absence of a computerized information system, some of the LMICs use ingenuous methods to collect aggregate performance data to monitor the programmes. However, due to the heterogeneity in the definition of the indicators and the method of estimating them, the data are often difficult to compare (Table 1) (18).
The CanScreen5 project will develop guidelines to improve data collection and evaluate the screening programmes using predefined indicators. The portal will have an e-learning platform to train the data providers and ensure harmonization of data collection across the countries. The dissemination of the qualitative and quantitative data collected from the countries will be through easily interpretable fact sheets (by countries and by cancer sites), interactive tables, maps and charts displayed on the CanScreen5 web platform. The programme managers will be able to identify the gaps and take corrective actions not only from the analysed data from their own programmes, but also from the comparative data submitted by the other countries.
Conclusion
The CanScreen5 platform is a freely accessible web-based platform designed to uniformly gather and store information on cancer screening programmes and initiatives across the globe and will reflect data from the real world. It provides the requisite data collection tools, the standardized methodology for estimating the performance indicators and the facility to compare the indicators with national and international standards. By providing a freely accessible platform to visualize the performance data analysed with a common set of indicators, CanScreen5 allows the cancer screening programmes to compare their performance over time and with other similar programmes. We expect this initiative to impact the capacity-building in monitoring and quality improvement of cancer screening programmes around the world.
Disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer / World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer / World Health Organization.