Ch Yip, Consultant Breast Surgeon, Ramsay Sime Darby Health Care, Kuala Lumpur, Malaysia, Clinical Professor, University Tunku Abdul Rahman, Kuala Lumpur, Malaysia and Visiting Consultant, University of Malaya, Kuala Lumpur, Malaysia
Breast cancer is the commonest cancer in most Asian countries, despite wide variations in ethnicity, culture and economics. The majority of Asian countries are classified as low- and middle-income, and breast cancer typically presents at a younger age and with later stages compared to their western counterparts. Lack of treatment facilities leads to poor survival, which can be improved with culturally appropriate, resource-stratified guidelines for early detection and optimal management of breast cancer.
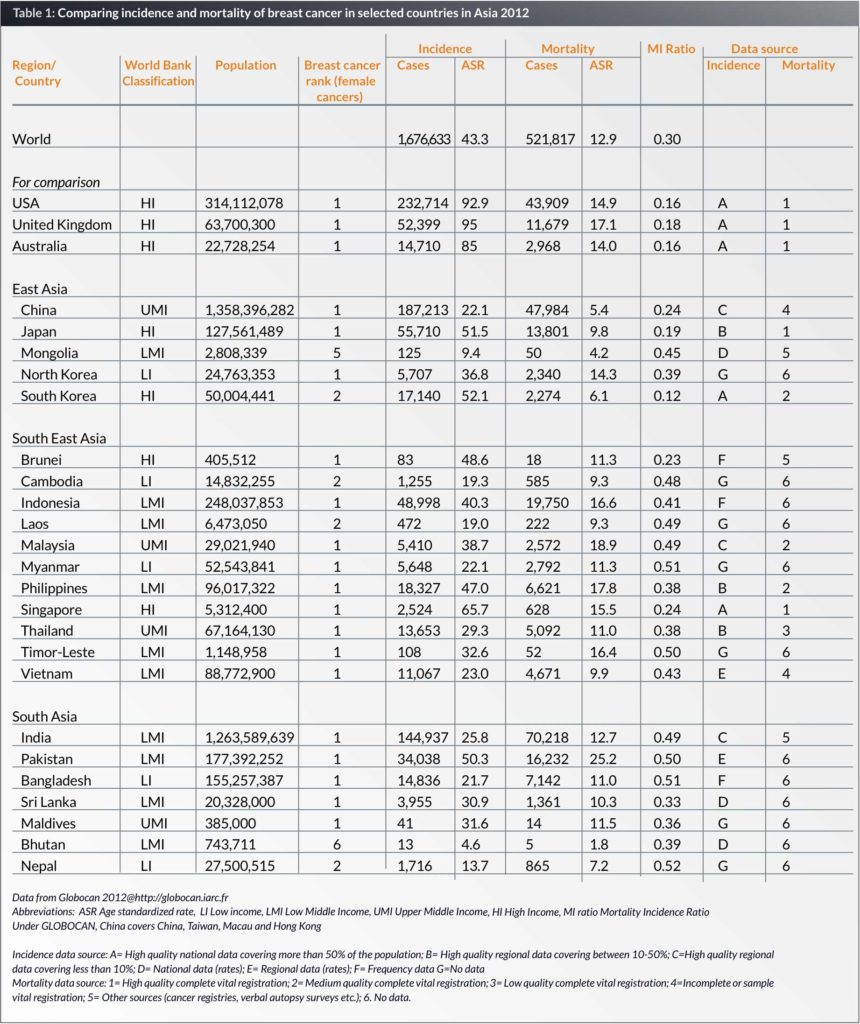
Asia is the world’s largest continent, comprising 48 countries and a population of 4.4 billion, which is 60% of the world’s population. Asia varies diversely within its regions with regards to ethnic groups, cultures, environments, economics, historical ties and government systems. This review will focus on three major regions in Asia, that is East Asia, South Asia and South East Asia, comprising 23 countries, and with a population of over 3.8 billion (Table 1).
Breast cancer is the commonest cancer in most parts of the world including Asia. Risk factors associated with breast cancer, incidence and mortality are well-studied in Europe and North America, where the age-standardized incidence rate (ASR) of breast cancer is double or even triple the ASR in most parts of Asia (1). However with the rapid transition from developing to developed countries, westernization of the traditional risk factors occurs. Women are becoming more emancipated, and exert control over their reproductive life; hence the modern Asian women have children later, have less children and breastfeed for shorter periods. Women are also entering the workforce and have a more sedentary lifestyle. With better nutrition and changes in lifestyle, the age at menarche is decreasing while the age at menopause is increasing. The traditional high fibre, soy-based diet, which is believed to be protective against breast cancer, is also changing to a more western-style diet. These changes in risk factors are believed to be the reason for the rapidly increasing ASR of breast cancer in Asia (2). In Singapore, the average annual increase in the incidence of breast cancer was 3.6% over a 25 year period from 1968 to 1992 (3), and this phenomenon is also seen in various parts of Asia (4). It is anticipated that breast cancer will emerge as a major heath issue for women in the Asian region in the near future. Data from Cancer Incidence in Five Continents noted that incidence rates in Asian women living in the United States was 1.5 to 4 times higher than the corresponding rate in the women’s respective countries of origin, which suggests that environmental factors play a role (5).
Incidence and mortality
Incidence and mortality estimates for the year 2012 were taken from the GLOBOCAN database compiled by the International Agency for Research on Cancer (IARC). Asia faces various problems in cancer registration such as inadequate quality, weak infrastructure and insufficient coverage (6). There is no available data from the low-income countries, such as North Korea, Cambodia, Laos, Nepal and Myanmar. Where there is no available data, incidence estimates are derived from neighbouring countries (Table 1).
The ASR of breast cancer in Western countries such as the United States, United Kingdom and Australia are 92.9, 95 and 85 per 100,000 respectively, more than double the rates in most of the Asian countries. The ASR in Asia varies widely, from 65.7 per 100,000 in Singapore to 4.6 per 100,000 in Bhutan. It is interesting to note that the ASR shows an increasing trend from the low-income to the high-income countries. What is noteworthy is that breast cancer is the commonest female cancer in 20 out of the 23 countries in this review (Table 1).
Even in the same country, the ASR can vary between different ethnic groups, as seen in Malaysia, where the ASR is highest in the Chinese (59.9 per 100,000), followed by the Indians (54.2 per 100,000), with the lowest rates in the Malays (34.9 per 100,000) (7). In addition, remarkable differences were seen in the age-specific incidence rates by ethnic groups in Singapore. After the age of 49 years old, the incidence rates for Malays and Chinese levelled off while in the Indians it continued to rise. This suggests that Chinese and Malay women born in later cohorts were at increased risk of developing breast cancer compared to their counterparts in the earlier cohorts (8). The relationship between multiparity, ethnicity and premenopausal breast cancer was studied in Singapore and found that while multiparity did not modify risk of premenopausal breast cancer in Chinese and Indians, there was a significant risk reduction in Malays with increasing parity (9). This suggests that risk factors may differ in different ethnicities.
The Mortality Incidence Ratio (MIR) is calculated by dividing the standardized mortality rate by the standardized incidence rate, and is a measure of prognosis after diagnosis (10). A low MIR is an indication of good survival. It is noted that the MIR is lower in the high-income countries, with the lowest MIR in South Korea (0.12) and the highest MIR is 0.52 in Nepal, a low-income country (Table 1). A study in a multiethnic country, Malaysia, showed that Malays have a significantly poorer survival from breast cancer, compared to the Chinese, and this was independent of stage at diagnosis and treatment. Differences in lifestyle, diet, coping skills and pharmacogenomics may explain these differences (11).
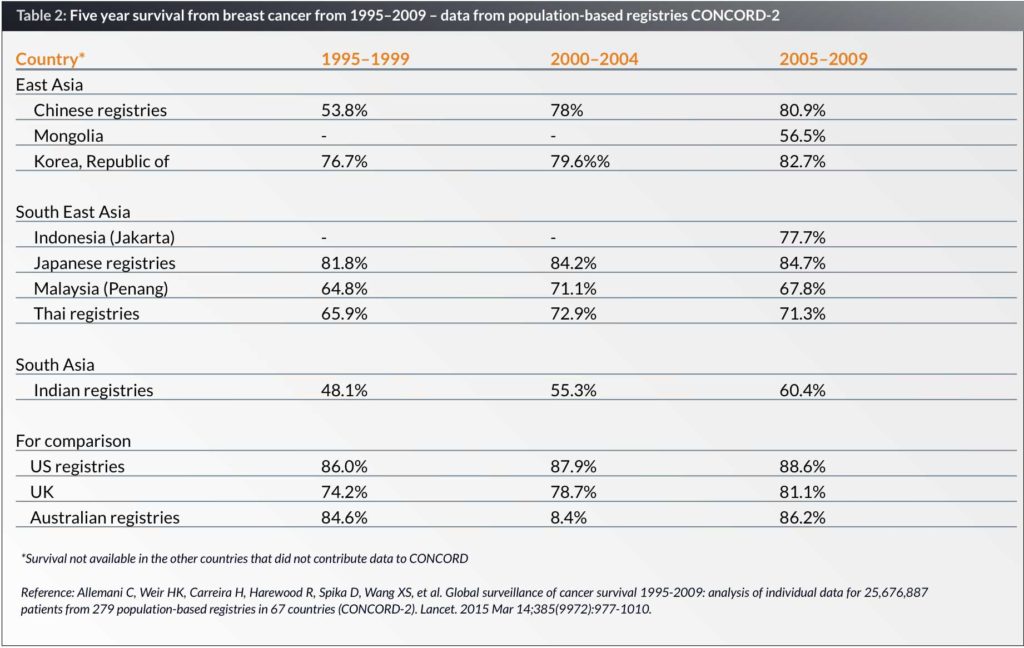
Global surveillance of cancer survival 1995–2009 from population-based registries in 67 countries showed that in all countries, breast cancer survival has improved over the three periods from 1995–1999, 2000–2004 and 2005–2009. In Asia, the best five-year survival is seen in Japan (84.75) and South Korea (82.7%) compared with the five-year survival of 67.8% in Malaysia, 60.4% in India and 56.5% in Mongolia. In comparison, the five-year survival rates in the United Kingdom, United States and Australia are 81.1%, 88.6% and 86.2% respectively (Table 2) (12).
Presentation of breast cancer in Asia
The mean age at presentation of breast cancer in Asia is around 50 years old; and this is about ten years younger than the mean age at presentation seen in Western countries (13). It is important to note that at every age group, the age specific incidence rate of breast cancer is still higher in the Western countries compared to Asia. However, the difference is not as large in the younger age groups compared to the older age groups (14). Hence the younger age at presentation may be due to the broad-based population age structure seen in developing countries, with a younger median age compared to developed countries: for example, the median age in South East Asia is 27.2 years compared with 43.2 years in Western Europe (15). Besides age demographics, a cohort effect is seen in most Asian countries, where women born in earlier cohorts that is, before the Second World War are at a lower risk compared to women born after the Second World War. This has been described in a study in Shanghai, where the incidence peak moved from the 40–44 year age group in the previous two decades to the 50–54 year age group in the most recent decade. The median age at diagnosis also increased from 47.5 years in 1990 to 50 years in 2007 (16).
In the United States, Asian-Americans are more likely to have the infiltrating ductal carcinoma subtype compared to American Whites and less likely to have the infiltrating lobular subtype, and they are more likely to be hormone receptor (estrogen receptor ER and progesterone receptor PR) negative (17). In Malaysia, Malays were found to have an independently lower rate of ER positive cancers (52%) compared to Chinese (59.4%) and Indians (55.1%) (18). A similar rate of ER positive tumours in the Chinese was also seen in Hong Kong (19). In Western countries, the rate of ER positive breast cancer has increased over time, from 75.4% in 1992 to 77.5% in 1998 (20). This increase is similar to that seen in Malaysia, where the ER positive rate increased by about 2% for every five-years cohort, from 54.5% in 1994–1998, to 56.4% in 1999–2003 and 58.4% in 2004–2008 (18). When we look at the incidence of ER positive breast cancer rather than the ER positive rate, it was found that the incidence of ER negative breast cancer remained constant while the incidence of ER positive breast cancer increased over time (20). Hence most of the increase in breast cancer incidence has been mostly in ER positive breast cancer. Breast cancer seen in rural areas are more likely to be ER negative compared to those in urban areas (21). In Asia, with increasing incidence and urbanization, the rate of ER positive breast cancers is likely to increase to similar rates as those seen in Western countries.
Based on the status of ER, PR and HER2 (human epidermal growth factor-2), there are four subtypes of breast cancer i.e., Luminal A (ER or PR +, HER2 -), Luminal B (ER or PR+, HER2 +), HER2 overexpressing (ER- PR- HER2+) and triple negative. (ER-PR-HER2-). The best survival is in those with the Luminal A subtype while the triple negative breast cancer (TNBC) subtype have the poorest survival. Western populations are more likely to have the Luminal A subtype when compared to Asians. The USA National Breast Cancer Database in the United States found that while TNBC was more common in American Blacks, Asians were more likely to have HER2 overexpressing breast cancer. Nulliparity, increasing age of first childbirth and obesity in younger women were risk factors for ER+ and PR+ tumours but not TNBC (22). A study of 1,034 cases of breast cancer in Sarawak, Malaysia, showed a wide variation in breast cancer subtypes within the multiethnic population, with a higher incidence of HER2 overexpression in Malays (29%) compared to Chinese (22%) while the rate of TNBC in the Sarawak natives was 37% compared to 23% in Chinese. Different risk factors in the different ethnicities may explain these differences (23).
Outcomes of breast cancer in Asia
The ultimate measure of outcome, which is survival, depends on two main factors – stage at diagnosis (which is dependent on size of tumour and axillary lymph node status) and access to optimal treatment. Tumour factors, such as the molecular type, are also important, while patient factors, such as age, ethnicity and socioeconomic status play a less important role.
Stage at diagnosis
Breast cancer is typically diagnosed in late stages, defined as Stage 3 and Stage 4, in the low- and middle-income countries (LMICs) in Asia. In Lucknow, India, 60% of breast cancers were late-staged, compared to 27% in Kuala Lumpur, Malaysia (24) and 52.2% in Kota Kinabalu, Sarawak (25). In contrast only 11.8% were diagnosed with late stages in Korea (26).
Besides geographical isolation, poverty and ignorance, there are several cultural and economic obstacles to early detection of breast cancer. Traditionally Asian women play a subservient role and are themselves care-givers, looking after children and taking care of the cooking and cleaning. Having breast cancer would lead to stigmatization, with the fear that a woman may be abandoned by her husband. Hence a woman is unlikely to disclose the presence of a painless breast lump until it begins to ulcerate. A study in Malaysia showed that belief in alternative therapy, cancer fatalism, poor recognition of symptoms as well as poor decision-making skills were responsible for delayed presentation, Women needed sanction by family members to seek medical treatment (27).
Access to treatment
Treatment for breast cancer involves a multidisciplinary approach and consists of surgery, radiotherapy, chemotherapy, hormone therapy and targeted therapy. The percentage of GDP (Gross Domestic Product) spent on health-care varies from 17% in the United States to 1.4% in Timor-Leste (28). LMICs typically spend an average of 3.5% of their GDP on health care, leading to a lack of manpower and infrastructure for optimal treatment of not just breast cancer but many other medical conditions. The majority of LMICs do not have universal health coverage, and hence out-of-pocket expenses are high leading to financial catastrophe and economic hardship. A recent study of eight LMICs in South-East Asia, the ACTION (Asean costs in oncology) study, found that at the end of one year, 48% had experienced financial catastrophe (defined as spending more than 30% of household income on health care) and 29% had died (29). Thirty-three percent of those who had no economic hardship at baseline experienced economic hardship at one year, of whom 45% were unable to pay for medicines (30).
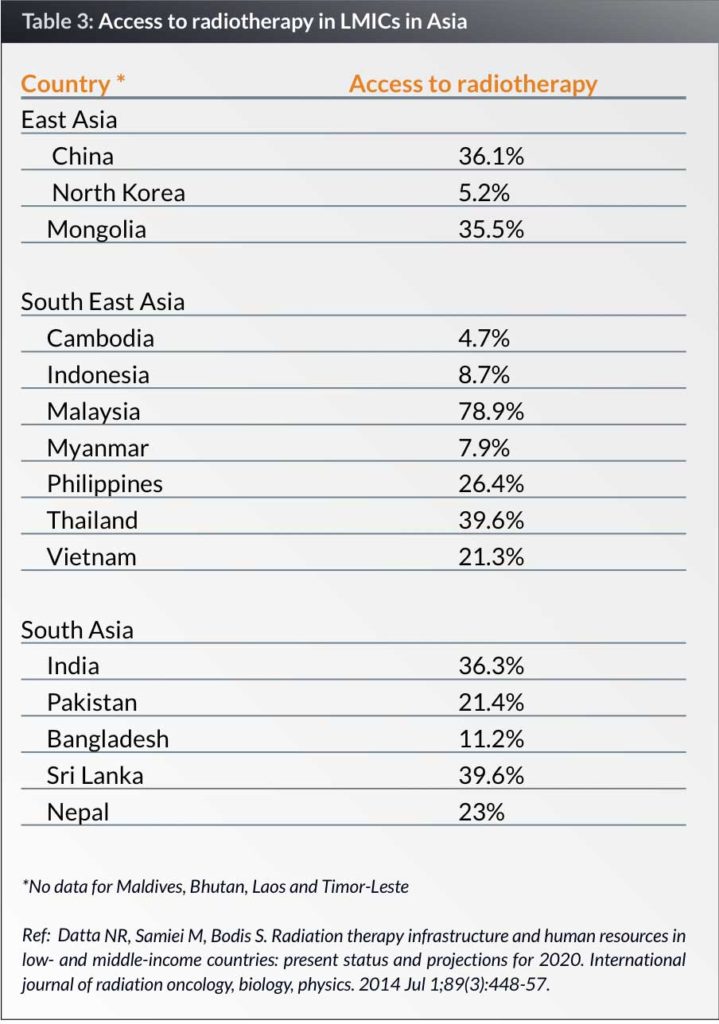
While surgical services are established even in low-resource settings, there is a lack of medical and radiation oncology services. Access to radiation therapy access ranges from 4.7% in Cambodia to 78.9% in the LMICs in the 23 Asian countries studied. Four of these countries (Maldives, Bhutan, Timor Leste and the Maldives) do not have radiotherapy units. (Table 3) (31).
Besides surgery and radiotherapy, chemotherapy, hormone therapy and targeted therapy has been shown to improve survival from breast cancer. The World Health Organization (WHO) published the 19th WHO model list of essential medicines in April 2015, which lists 46 anticancer agents considered essential for a basic health-care system. This list includes 11 anticancer drugs indicated for treatment in early or metastatic breast cancer. (cyclophosphamide, docetaxel, doxorubicin, fluorourael, methotrexate, paclitaxel, trastuzumab, vinorelbine, anastrazole, tamoxifen, leuproretin). In addition there are two more agents, cisplatin and gemcitabine, where breast cancer is not listed as an indication, but can also be used for breast cancer (32).
The National Essential Medicine List (NEML) from 75 LMICs (of which 10 were in the Asian countries in this review) were studied, and it was found that less than 10% of HER2 targeted therapy, 12% of aromatase inhibitors and 28% of taxanes were incorporated in the NEMLS, compared to 71–78% of tamoxifen and first line chemotherapy regimes (anthracycline- based and CMF regimes) (33). Since trastuzumab, aromatase inhibitors and taxanes are the more expensive anticancer agents, it is not surprising that 45% of patients who experienced economic hardship in the ACTION study had to stop their medicines. A study in a middle-income country on survival in the different molecular subtypes of breast cancer showed that without trastuzumab for HER2 overexpressing breast cancer, survival was similar in the HER2 overexpressing and the TNBC (34).
Strategies to improve outcomes in LMICs in Asia
Biological and patient factors are not modifiable, hence early detection and optimal access to treatment are important strategies to improve outcomes in LMICs. The Breast Health Global Initiative (BHGI) is an alliance
of individuals, governmental and non-governmental organizations that develop guidelines for early detection and treatment of breast cancer in LMICs with the goal of improving outcomes. These guidelines are stratified by resource levels, that is, basic, limited, advanced and maximal, such that even with the most basic resources, women can have access to treatment (35).
Early detection in LMICs is more about down-staging clinical disease rather than looking for asymptomatic disease with screening mammography (36, 37). Randomized clinical trials on screening mammography have not been conducted in Asian countries to determine whether it can reduce mortality from breast cancer. However various opportunistic mammographic screening programmes in Asia have determined a cancer detection rate of 0.5% in women aged 40–70 years old (38, 39). The detection rate in women younger than 50 years old is much lower, and hence screening below 50 years old is unlikely to make any difference to mortality, especially when the prevalent age group for breast cancer in Asia is 40–49 years old (38). Therefore early detection with a combination of clinical breast examination and breast self-examination is likely to downsize tumours. Unfortunately RCTs on CBE and BSE have not been shown to reduce mortality from breast cancer (40, 41) although down-staging with CBE has been demonstrated (42). Public awareness and education on signs and symptoms of breast cancer and the benefit of early detection is an important first step to implementing an early detection programme. It is also important to determine how receptive women are to an early detection programme before proceeding, as seen in the Philippines where a CBE programme failed because 42% of women detected with a breast lump refused any further investigations (43). A mammogram with CBE programme in Jakarta Indonesia also failed because 42.8% of women who were diagnosed with breast cancer refused treatment (44). Barriers to early detection and treatment need to be identified, and treatment facilities need to be developed before any early detection programme (45).
Surgery plays a pivotal role in breast cancer treatment, whether as a curative or palliative procedure. It is also considerably cheaper and requires less resources than radiotherapy or systemic therapy. In low-resource settings with no access to radiotherapy, a modified radical mastectomy alone can be curative in early stage disease. Breast conserving surgery should not be carried out unless radiotherapy is available (46). However, breast cancer in LMICs typically present with advanced disease, where mastectomy needs to be combined with chemotherapy and radiotherapy. Whether as adjuvant therapy or as primary therapy, chemotherapy requires more resources, and in low-resource settings can be administered by surgeons or general physicians in areas where there are no oncologists (47). The WHO List of Essential Medicines for breast cancer is adequate to provide optimal treatment for breast cancer, and as the majority of the drugs are no longer under patency, the generic forms should be cheap enough for LMICs to purchase. Delivery of radiotherapy is limited by its availability; however there have been models of public hospitals purchasing radiotherapy services from private medical centres where it is available, and indeed, even purchasing services from outside the country. While each country should develop guidelines suitable for their needs, the BHGI guidelines provide a good template for the management of breast cancer. Strong coordination of surgical and oncological services is required to provide optimal outcomes with good quality of life.
Conclusion
Whether breast cancer is the same disease in Asia compared to Western countries requires further research (48). The incidence of breast cancer in Asia is increasing and the incidence is highest in the higher income Asian countries. The age of onset is also noted to be increasing in Asia and may very likely approach that of the western countries. The subtypes of breast cancer may be related to epidemiological risk factors, particularly the reproductive risk factors, rather than genetic differences, and as risk factors change in Asia, the geographical differences in subtypes may also become less significant. Hence as the countries in Asia transition from low income to high income, a process which may take several generations, breast cancer in Asia may also evolve to be similar to Western countries.
Outcomes depend on early detection and optimal treatment, and even when Asian countries can afford to develop early detection programmes and provide optimal treatment, public education is important to overcome barriers to early detection and treatment. Only then will survival from breast cancer improve to be comparable with the more developed nations.
Biography
References
1. GLOBOCAN 2012 at http://globocan.iarc.fr.
2. Porter P. “Westernizing” women’s risks? Breast cancer in lower-income countries. The New England journal of medicine. 2008 Jan 17;358(3):213-6. PubMed PMID: 18199859.
3. Seow A, Duffy SW, McGee MA, Lee J, Lee HP. Breast cancer in Singapore: trends in incidence 1968-1992. International journal of epidemiology. 1996 Feb;25(1):40-5. PubMed PMID: 8666502.
4. Shin HR, Joubert C, Boniol M, Hery C, Ahn SH, Won YJ, et al. Recent trends and patterns in breast cancer incidence among Eastern and Southeastern Asian women. Cancer causes & control : CCC. 2010 Nov;21(11):1777-85. PubMed PMID: 20559704.
5. Forman D, Bray F, Brewster DH eta;, editors. Cancer Incidence in Five Continentsm Vol X (electronic version). Lyon: IARC, 2013.
6. Sobue T, Inoue M, Tanaka H. Cancer Registry and Epidemiological Study Working Group report. Japanese journal of clinical oncology. 2010 Sep;40 Suppl 1:i76-81. PubMed PMID: 20870925.
7. Lim GCC RS, Halimah Y. (Eds.) Cancer Incidence in Peninsular Malaysia 2003-2005. National Cancer Registry. Kuala Lumpur 2008.
8. Sim X, Ali RA, Wedren S, Goh DL, Tan CS, Reilly M, et al. Ethnic differences in the time trend of female breast cancer incidence: Singapore, 1968-2002. BMC cancer. 2006;6:261. PubMed PMID: 17078893. PubMed PMID: 1636657.
9. Verkooijen HM, Yap KP, Bhalla V, Chow KY, Chia KS. Multiparity and the risk of premenopausal breast cancer: different effects across ethnic groups in Singapore. Breast cancer research and treatment. 2009 Feb;113(3):553-8. PubMed PMID: 18311581.
10. Hebert JR, Daguise VG, Hurley DM, Wilkerson RC, Mosley CM, Adams SA, et al. Mapping cancer mortality-to-incidence ratios to illustrate racial and sex disparities in a high-risk population. Cancer. 2009 Jun 1;115(11):2539-52. PubMed PMID: 19296515. Pubmed Central PMCID: 2688832.
11. Bhoo-Pathy N, Hartman M, Yip CH, Saxena N, Taib NA, Lim SE, et al. Ethnic differences in survival after breast cancer in South East Asia. PloS one. 2012;7(2):e30995. PubMed PMID: 22363531. Pubmed Central PMCID: 3283591.
12. Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015 Mar 14;385(9972):977-1010. PubMed PMID: 25467588. Pubmed Central PMCID: 4588097.
13. Leong SP, Shen ZZ, Liu TJ, Agarwal G, Tajima T, Paik NS, et al. Is breast cancer the same disease in Asian and Western countries? World journal of surgery. 2010 Oct;34(10):2308-24. PubMed PMID: 20607258. Pubmed Central PMCID: 2936680.
14. Ghiasvand R, Adami HO, Harirchi I, Akrami R, Zendehdel K. Higher incidence of premenopausal breast cancer in less developed countries; myth or truth? BMC cancer. 2014;14(1):343. PubMed PMID: 24884841. Pubmed Central PMCID: 4032450.
15. Median Age at http://data.un.org/Data.aspx?d=PopDiv&f=variableID%3A41.
16. Fan L, Zheng Y, Yu KD, Liu GY, Wu J, Lu JS, et al. Breast cancer in a transitional society over 18 years: trends and present status in Shanghai, China. Breast cancer research and treatment. 2009 Sep;117(2):409-16. PubMed PMID: 19153831.
17. Li CI, Malone KE, Daling JR. Differences in breast cancer hormone receptor status and histology by race and ethnicity among women 50 years of age and older. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2002 Jul;11(7):601-7. PubMed PMID: 12101106.
18. Yip CH, Pathy NB, Uiterwaal CS, Taib NA, Tan GH, Mun KS, et al. Factors affecting estrogen receptor status in a multiracial Asian country: an analysis of 3557 cases. Breast. 2011 Apr;20 Suppl 2:S60-4. PubMed PMID: 21349715.
19. Chow LW, Ho P. Hormonal receptor determination of 1,052 Chinese breast cancers. Journal of surgical oncology. 2000 Nov;75(3):172-5. PubMed PMID: 11088048.
20. Li CI, Daling JR, Malone KE. Incidence of invasive breast cancer by hormone receptor status from 1992 to 1998. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2003 Jan 1;21(1):28-34. PubMed PMID: 12506166.
21. Dey S, Soliman AS, Hablas A, Seifeldin IA, Ismail K, Ramadan M, et al. Urban-rural differences in breast cancer incidence by hormone receptor status across 6 years in Egypt. Breast cancer research and treatment. 2010 Feb;120(1):149-60. PubMed PMID: 19548084. Pubmed Central PMCID: 2808467.
22. Sineshaw HM, Gaudet M, Ward EM, Flanders WD, Desantis C, Lin CC, et al. Association of race/ethnicity, socioeconomic status, and breast cancer subtypes in the National Cancer Data Base (2010-2011). Breast cancer research and treatment. 2014 Jun;145(3):753-63. PubMed PMID: 24794028.
23. Devi CR, Tang TS, Corbex M. Incidence and risk factors for breast cancer subtypes in three distinct South-East Asian ethnic groups: Chinese, Malay and natives of Sarawak, Malaysia. International journal of cancer Journal international du cancer. 2012 Dec 15;131(12):2869-77. PubMed PMID: 22407763.
24. Agarwal G, Pradeep PV, Aggarwal V, Yip CH, Cheung PS. Spectrum of breast cancer in Asian women. World journal of surgery. 2007 May;31(5):1031-40. PubMed PMID: 17387549.
25. Leong BD, Chuah JA, Kumar VM, Yip CH. Breast cancer in Sabah, Malaysia: a two year prospective study. Asian Pacific journal of cancer prevention: APJCP. 2007 Oct-Dec;8(4):525-9. PubMed PMID: 18260722.
26. Kim Z, Min SY, Yoon CS, Lee HJ, Lee JS, Youn HJ, et al. The basic facts of korean breast cancer in 2011: results of a nationwide survey and breast cancer registry database. Journal of breast cancer. 2014 Jun;17(2):99-106. PubMed PMID: 25013429. Pubmed Central PMCID: 4090324.
27. Taib NA, Yip CH, Low WY. A Grounded Explanation of Why Women Present with Advanced Breast Cancer. World journal of surgery. 2013 Nov 27. PubMed PMID: 24280975.
28. The World Bank at http://data.worldbank.org.
29. Group AS, Kimman M, Jan S, Yip CH, Thabrany H, Peters SA, et al. Catastrophic health expenditure and 12-month mortality associated with cancer in Southeast Asia: results from a longitudinal study in eight countries. BMC medicine. 2015;13:190. PubMed PMID: 26282128. Pubmed Central PMCID: 4539728.
30. Bhoo-Pathy N. Prioritising strategies to addess the economic impact of cancer in South East Asia. Presented at ESMO Asia 2015 Dhccc.
31. Datta NR, Samiei M, Bodis S. Radiation therapy infrastructure and human resources in low- and middle-income countries: present status and projections for 2020. International journal of radiation oncology, biology, physics. 2014 Jul 1;89(3):448-57. PubMed PMID: 24751411.
32. http://www.who.int/medicines/publications/essentialmedicines/EML2015_8-May-15.pdf WLoEMa.
33. Bazargani YT, de Boer A, Schellens JH, Leufkens HG, Mantel-Teeuwisse AK. Essential medicines for breast cancer in low and middle income countries. BMC cancer. 2015;15:591. PubMed PMID: 26283654. Pubmed Central PMCID: 4538762.
34. Subramaniam S, Bhoo-Pathy N, Taib NA, Tan GH, See MH, Jamaris S, et al. Breast Cancer Outcomes as Defined by the Estrogen Receptor, Progesterone Receptor, and Human Growth Factor Receptor-2 in a Multi-ethnic Asian Country. World journal of surgery. 2015 Oct;39(10):2450-8. PubMed PMID: 26138872.
35. Anderson BO, Yip CH, Smith RA, Shyyan R, Sener SF, Eniu A, et al. Guideline implementation for breast healthcare in low-income and middle-income countries: overview of the Breast Health Global Initiative Global Summit 2007. Cancer. 2008 Oct 15;113(8 Suppl):2221-43. PubMed PMID: 18816619.
36. Burton R, Bell R. The global challenge of reducing breast cancer mortality. The oncologist. 2013;18(11):1200-2. PubMed PMID: 24218000. Pubmed Central PMCID: 3825305.
37. Corbex M, Burton R, Sancho-Garnier H. Breast cancer early detection methods for low and middle income countries, a review of the evidence. Breast. 2012 Aug;21(4):428-34. PubMed PMID: 22289154.
38. Teh YC, Tan GH, Taib NA, Rahmat K, Westerhout CJ, Fadzli F, et al. Opportunistic mammography screening provides effective detection rates in a limited resource healthcare system. BMC cancer. 2015;15:405. PubMed PMID: 25972043. Pubmed Central PMCID: 4437679.
39. Lui CY, Lam HS, Chan LK, Tam KF, Chan CM, Leung TY, et al. Opportunistic breast cancer screening in Hong Kong; a revisit of the Kwong Wah Hospital experience. Hong Kong medical journal = Xianggang yi xue za zhi / Hong Kong Academy of Medicine. 2007 Apr;13(2):106-13. PubMed PMID: 17406037.
40. Thomas DB, Gao DL, Ray RM, Wang WW, Allison CJ, Chen FL, et al. Randomized trial of breast self-examination in Shanghai: final results. Journal of the National Cancer Institute. 2002 Oct 2;94(19):1445-57. PubMed PMID: 12359854.
41. Mittra I, Mishra GA, Singh S, Aranke S, Notani P, Badwe R, et al. A cluster randomized, controlled trial of breast and cervix cancer screening in Mumbai, India: methodology and interim results after three rounds of screening. International journal of cancer Journal international du cancer. 2010 Feb 15;126(4):976-84. PubMed PMID: 19697326.
42. Sankaranarayanan R, Ramadas K, Thara S, Muwonge R, Prabhakar J, Augustine P, et al. Clinical breast examination: preliminary results from a cluster randomized controlled trial in India. Journal of the National Cancer Institute. 2011 Oct 5;103(19):1476-80. PubMed PMID: 21862730.
43. Pisani P, Parkin DM, Ngelangel C, Esteban D, Gibson L, Munson M, et al. Outcome of screening by clinical examination of the breast in a trial in the Philippines. International journal of cancer Journal international du cancer. 2006 Jan 1;118(1):149-54. PubMed PMID: 16049976.
44. Kardinah D, Anderson BO, Duggan C, Ali IA, Thomas DB. Short report: Limited effectiveness of screening mammography in addition to clinical breast examination by trained nurse midwives in rural Jakarta, Indonesia. International journal of cancer Journal international du cancer. 2014 Mar 1;134(5):1250-5. PubMed PMID: 24037942.
45. Yip CH, Smith RA, Anderson BO, Miller AB, Thomas DB, Ang ES, et al. Guideline implementation for breast healthcare in low- and middle-income countries: early detection resource allocation. Cancer. 2008 Oct 15;113(8 Suppl):2244-56. PubMed PMID: 18837017.
46. Eniu A, Carlson RW, El Saghir NS, Bines J, Bese NS, Vorobiof D, et al. Guideline implementation for breast healthcare in low- and middle-income countries: treatment resource allocation. Cancer. 2008 Oct 15;113(8 Suppl):2269-81. PubMed PMID: 18837019.
47. Dare A AB, Sullivan R, Pramesh CS, Yip CH, Ilbawi A, Adewole J, Badwe R, and Gavreau C. Surgical Services for Cancer Care. Cancer: Disease Control Priorities, Third Edition Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2015 Apr 02. Chapter 13.
48. Bhoo-Pathy N, Yip CH, Hartman M, Uiterwaal CS, Devi BC, Peeters PH, et al. Breast cancer research in Asia: adopt or adapt Western knowledge? European journal of cancer. 2013 Feb;49(3):703-9. PubMed PMID: 23040889.