Hellen Gelband (Left), Associate Director For Policy, Center For Disease Dynamics, Economics And Policy, Susan Horton (Center Left), Chair, Centre For International Governance Innovation, Balsillie School Of International Affairs, University Of Waterloo, USA, Rengaswamy Sankaranarayanan (Center right), Special Adviser On Cancer Control and Head of the Screening Group, International Agency For Research on Cancer, France and Prabhat Jha (Right), Professor In Global Health And Epidemiology, University Of Toronto, Canada
Cancer (volume 3 of Disease Control Priorities, third edition) is a guide for low- and middle-income countries to advance cancer control through the middle of the twenty-first century. DCP3 Cancer offers the concept of an “essential package” of interventions that spans prevention, diagnosis, treatment and palliative care to address a significant portion of a country’s cancer burden effectively, cost-effectively, feasibly and affordably, and which will help achieve the new Sustainable Development Goals for noncommunicable diseases.
Cancer, volume 3 of Disease Control Priorities, third edition (DCP3), is a guide for low- and middle-income countries (LMICs) to help advance cancer control through the middle of the twenty-first century (1). It provides the means to help meet the new Sustainable Development Goals (2) and provides a blueprint for expanding cancer control as a component of universal health care. DCP3 Cancer is a collaborative effort of 79 authors contributing to 18 chapters on the cancer burden in LMICs, specific cancers, treatment modalities, financing and economics.
Using the criteria of addressing a significant portion of the cancer burden, intervention effectiveness and cost-effectiveness, feasibility and national affordability, DCP3 Cancer offers the idea of an “essential package” of interventions that spans prevention, diagnosis, treatment and palliative care. While not a formal part of the package, well-functioning cancer registries and context-relevant research are also essential.
The pattern of cancers and the existing capacity for cancer control — and therefore the needs of countries — vary tremendously around the world. As a first rough cut, DCP3 Cancer distinguishes among low-income, lower-middle income and upper-middle income, according to World Bank definitions based on per capita gross domestic product (GDP).
A “model” package is provided as a guide for countries to consider when tailoring a package to national needs. An important point is that, even for the poorest countries, some treatment of early-stage cancers that involves surgery, drug therapy (chemotherapy and/or endocrine therapy) and radiotherapy, should be included along with preventive interventions, even if initially at only a single centre of excellence in a major city. Palliative care with a foundation of pain control using opioid drugs (the most basic being oral morphine) should be made widely available at least to those with end-stage cancer pain down to village level, as a near-term priority.
In developing the essential package, cost-effectiveness estimates were compiled for each cancer and each intervention, from studies in or including low- and middle-income countries (LMICs), published in 2003–2013 (3), when possible. Such literature is scarce, however, and the literature was supplemented with studies from high-income countries.
All analyses were stratified by World Bank country group classifications as defined by 2013 per capita gross national income: 34 low-income countries (less than US$ 1,045), 50 lower-middle-income countries (US$ 1,046 to US$ 4,125), and 55 upper-middle-income countries (US$ 4,126 to US$ 12,745) (4).
Interventions were defined as “very cost-effective,” “cost-effective,” and “cost-ineffective” using the scale developed by the Commission on Macroeconomics and Health (2001) costing, respectively, < 1, 1–3, and > 3 times per capita income per QALY (or other measure) (5). The essential package includes interventions rated as very cost-effective and cost-effective and considered potentially affordable and feasible in resource-constrained environments.
The essential package is offered as a starting point for countries to consider what would be most useful within their borders. A country-specific package should respond not only to disease burden and resources, but also to the societal norms of the population as regards cancer. Whether or not a programme can be implemented is modulated by social, ethnic and cultural factors and beliefs, as well as the population’s education level, gender attitudes, and other factors. These considerations are left to individual country health-care policymakers.
Key Messages
Cancer is already a major cause of death in low- and middle-income countries (LMICs), particularly in middle-income countries, and will increase as a percentage of deaths in all LMICs, driven by population ageing and faster declines in other causes of death.
In most populations, helping current tobacco users to quit and young people not to start smoking are the most urgent priorities in cancer prevention (and also to control other noncommunicable diseases), along with vaccination against hepatitis B (HBV) and the human papillomavirus (HPV). Higher tobacco taxes and accompanying interventions will reduce cancer incidence and generate substantial extra revenues for governments.
Other than tobacco- and virus-related cancers, however, most of the increase in cancer incidence is not currently preventable, but many cases of cancer can be effectively treated. Early breast cancer and cervical cancer are common, and often curable; precancerous cervical lesions are even more curable. Childhood cancers are relatively rare, but many are highly curable.
In most LMICs, it will take years or decades to develop a workforce capable of treating the cancer patients who can be helped. Even with state-of-the-art treatment (as in many high-income countries today), a large proportion of treated patients will die painful deaths from cancer. A final recommendation of Cancer is widespread availability of opioid drugs, at least for end-of-life cancer pain. Currently, opioid access is absent in nearly all LMICs.
Box 1: The Disease Control Priorities series
DCP2, published in 2006, updated and extended DCP1 in several respects, including explicit consideration of the implications for health systems of expanded intervention coverage (20).
DCP3, which consists of nine topic-related volumes, extends and consolidates the concepts of platforms and packages, introduced in DCP2. It gives explicit consideration to the financial risk protection objective of health systems, including the distribution across income groups of the financial and health outcomes resulting from specific policies. The broad aim of DCP3 is to offer, for consideration and adaptation, essential intervention packages – such as the essential cancer package in this volume – and their related delivery platforms.
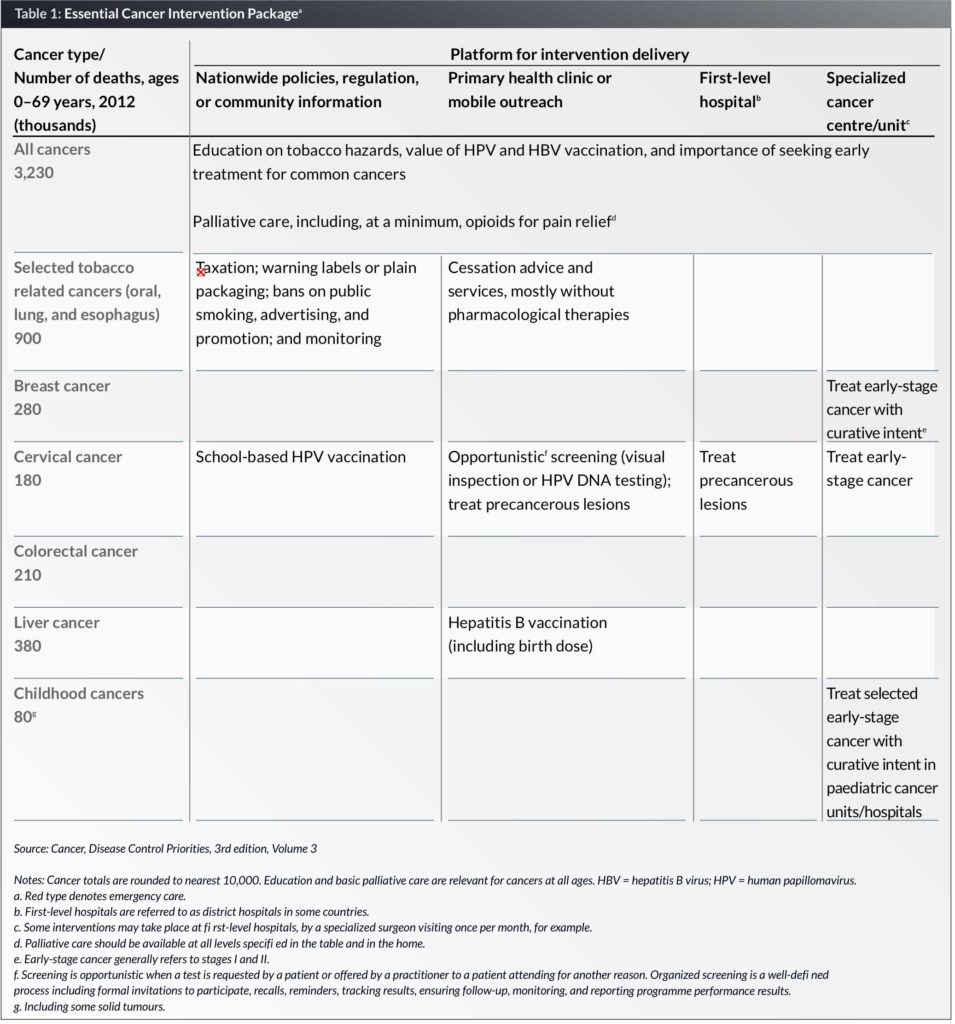
The DCP3 Cancer essential package of cost-effective and feasible interventions (Table 1) would, if fully implemented worldwide in LMICs, cost an additional US$ 20 billion per year, or 3% of total public spending on health in LMICs; 2.6% in upper-middle-income countries (UMICs); and 5% in lower-middle-income countries; but 13% in low-income countries (LICs). In per capita terms, this would cost US$ 5.70, US$ 1.70, and US$ 1.70 annually in UMICs, lower-middle-income, and LICs, respectively. Such increases are potentially feasible without external support in all but the LICs.
Cancer services that are considered appropriate for a national cancer strategy should be covered through universal health coverage as soon as countries are able to do so.
Global initiatives for cancer control in LMICs are needed to lower the costs of key inputs for the essential package, including large-scale commodity purchases; to expand technical assistance; and to promote cancer research.
The DCP3 Model Essential Cancer Package
The World Health Organization (WHO) produced a list of noncommunicable disease (NCD) best buys for LMICs in 2011, which were limited to services considered feasible at the primary care level. Those most relevant to cancer, which are included in the DCP3 Cancer package, are three preventive measures:
- a set of tobacco control interventions;
- hepatitis B vaccination to prevent liver cancer; and
- some form of screening and treatment for precancerous cervical lesions (6).
- The DCP3 Cancer essential package adds:
- HPV vaccination to prevent cervical cancer;
- treatment of early-stage cervical cancer;
- diagnosis and treatment for early breast cancer;
- diagnosis and treatment for selected, highly curable childhood cancers; and
- palliative care, including, at a minimum, opioid drugs for severe pain control.
Treating early stage breast and cervical cancer includes quality surgery, which could also be available for many other early-stage resectable cancers.
Each component of the essential package implies a range of interventions, the specifics of which may vary depending on the resources and infrastructure of each country. Resource-level appropriateness is a useful concept for deciding at a country level what will and will not be supported. The idea has been developed by the Breast Global Health Initiative, which has developed and refined it specifically for breast cancer over the last decade (7). It is grounded in the fact that several generations of effective breast cancer treatments exist, which differ not only in cost, but in the infrastructure needed to support them and the skill level of practitioners to apply them (see Box 2). The resource-level appropriate concept has gained adherents and groups have begun to apply it to a number of other common cancers with a range of effective treatments.
Box 2: Strategies for treating early breast cancer in LMICs
After technically successful surgery, treatments can be based on estrogen-receptor (ER) status, estimated recurrence risk, and general health (7). The ER status of surgically removed breast cancers can be determined relatively inexpensively (for about US$ 10, in India). If the cancer is ER-positive, about five years of endocrine drug therapy substantially reduces the 15-year recurrence risk and is relatively nontoxic. Endocrine drugs, such as tamoxifen or, for post-menopausal women, an aromatase inhibitor (AI) (22), can be dispensed safely to outpatients and are available as relatively low-cost generics (although even generic tamoxifen costs about US$ 15 per year in India, and generic AIs currently cost about US$ 50 per year). Chemotherapy also reduces recurrence but is more toxic and requires more careful medical supervision to ensure safety and efficacy. New on-patent drugs, for example, trastuzumab, that target other breast cancer receptors are not at present cost-effective in LMICs.
Relatively simple regimens of generic cytotoxic drugs (for example, four cycles of doxorubicin and cyclophosphamide with drug costs of about US$ 200 in India) should be practicable wherever surgery is practicable (7), and could be offered to women who are otherwise in good health but whose disease has already spread from the breast to the local lymph nodes (22). More effective cytotoxic regimens (for example, with taxanes) would increase toxicity, drug costs, and supervision costs.
In high-income countries, most women receiving appropriate treatment for early breast cancer survive their disease (22). The success rate of breast-conserving surgery (lumpectomy) plus radiotherapy to the conserved breast is about the same as for mastectomy (removal of the entire breast, and some local lymph nodes, if involved) and either approach can be offered, if safe radiotherapy is available. The most basic surgical procedure for stage II breast cancer is some form of mastectomy (7).
Costs of interventions
The cost of cancer interventions is seldom discussed explicitly, and documentation of actual costs is almost non-existent in the literature from LMICs. DCP3 Cancer reports a best estimate of per capita and global costs for the elements of the model essential package, for low-income, lower-middle-income, and upper-middle income countries. We stacked the direct costs of each intervention, then added a multiplier equal to 50% of the total to account for system costs (e.g., pathology, administration), as has been done in studies costing other health interventions, such as nutrition (8) and health systems (9).
In low-income and lower-middle-income countries, the package cost comes to less than US$ 2 per capita, and for upper-middle-income countries, US$ 5– US$ 6 per capita. Globally, the annual cost for LMICs is about US$ 20 billion in 2013 dollars. These must be taken as very rough estimates only, and countries must examine costs in their own systems before committing to provide these or other services. To the extent that such data are collected, both the data and the methods used would make valuable contributions to the global literature.
A useful metric is the cost of the package as a proportion of current total public spending on health. This is 2.6% in upper-middle-income countries, 5% in lower-middle-income countries, and 13% in LICs. By comparison, high-income countries devote 3–7% of their total health spending to cancer control (10). Most LMICs allocate far less; cancer currently accounts for about 1% of health spending (public and private) in Brazil and India, and 2% in China and Mexico (11–13).
Cost-effectiveness of interventions
The very scarce evidence for both effectiveness and costs signals a weak ability to estimate the cost-effectiveness (CE) of interventions. Using all available cost-effectiveness studies from LMICs and some from high-income countries, DCP3 Cancer offers a starting point for considering the cost-effectiveness of tobacco taxation; breast cancer treatment; liver cancer prevention; and cervical cancer prevention (with the HPV vaccine), screening and treatment. The remaining elements are not represented in this literature. The results are summarized in Table 2.
Affordability and financing
Financing for cancer control will have to come mainly from national health-care budgets, particularly in middle-income countries, where incomes are expected to continue rising. These are also countries that are beginning or expanding public financing for health (14,15). South Africa, for example, has assessed which interventions it might include in an expanded national health insurance package (16) and similar work is underway in India (9,17). In LICs, shifting enough health-care spending to fully fund expanded cancer control will take longer, but can proceed at a reasonable pace with some added support from global sources.
Global community
Finally, global initiatives might well help to lower the cost of cancer drugs and other commodities, and develop and disseminate standardized resource-appropriate treatment protocols, such as those developed by the Breast Health Global Initiative. Gavi, the Vaccine Alliance is a good example of how this has worked to increase vaccine coverage and reduce the cost of vaccines. Programmes to lower the cost of commodities for HIV/AIDS is another (18).
In addition to policy inputs by governments and international organizations, many cancer centres mainly in high-income countries – but including some in LMICs – run global programmes that maintain ongoing relationships with hospitals and centres in one or more LMICs. For example, the main global activity of the Fred Hutchinson Cancer Center in Seattle, Washington, is a close 20-year long relationship with the Uganda Cancer Institute (UCI) in Kampala. As a result of this collaboration, the UCI-Fred Hutch Cancer Center opened in May 2015. The center is a new US$ 10 million facility to serve Uganda and neighbouring countries in East Africa, where almost no cancer facilities exist. This and similar relationships can involve staff exchanges, training, telemedicine and other services, in addition to subsidizing buildings and equipment. These substantial contributions should be aligned with national needs.
Conclusion
The burden that cancer places on LMICs is increasing and will continue to do so throughout this century. Developing the infrastructure and workforce to meet the cancer challenge has been neglected by much of the global community – including the LMICs themselves, international organizations and the global health donor community. DCP3 Cancer provides a guide for LMICs that uses the best available evidence to develop cancer services beginning immediately and expanding over the next several decades.
Biographies
Professor Susan Horton is Professor at the University of Waterloo and holds the Centre for International Governance Innovation Chair in Global Health Economics in the Balsillie School of International Affairs there. She has consulted for the World Bank, the Asian Development Bank, several United Nations agencies, and the International Development Research Centre, among others in work carried out in over 20 low- and middle-income countries. She led the work on nutrition for the Copenhagen Consensus in 2008, when micro-nutrients were ranked as the top development priority.
Dr Rengaswamy Sankaranarayanan, after working in clinical oncology and cancer control in India, joined the International Agency for Research on Cancer in 1993, where he is Special Adviser on Cancer Control and Head of the Screening Group. His focus is research, training, programme development and technical assistance in early detection and cancer control, particularly in low- and medium-resourced countries. Dr Sankaranarayanan has taught in more than 50 international courses on cervical cancer screening, colposcopy, diagnosis and treatment, cancer registry epidemiology and cancer control; and provided technical support to more than 30 national cancer programmes.
Professor Prabhat Jha OC, MD, DPhil, FCAHS is an Endowed Professor in Global Health and Epidemiology, University of Toronto; Canada Research Chair, Dalla Lana School of Public Health; and founding Director, Centre for Global Health Research. He leads the Million Death Study in India, quantifying causes of mortality in over 2 million homes. His studies on tobacco control have enabled a global treaty signed by over 180 countries. He founded the Statistical Alliance for Vital Events, which focuses on reliable measurement of premature mortality worldwide. Jha is Officer of the Order of Canada (2012) and earned degrees from the University of Manitoba and Oxford University.
References
1. Gelband H, Jha P, Sankaranarayanan R, Horton S, eds. Cancer. In: Disease Control Priorities. 3rd ed. Washington, DC: The World Bank; 2015.
2. United Nations. Transforming our World: The 2030 Agenda for Sustainable Development. New York, NY: United Nations; 2015. Report No.: A/RES/70/1.
3. Horton S, Gauvreau CL. Cancer in Low-and Middle-Income Countries: An Economic Overview. In Disease Control Priorities: Volume 3, Cancer, edited by H. Gelband, P. Jha, R. Sankaranarayanan, and S. Horton. 3rd ed. Washington, DC: World Bank.
4. World Bank. Country and Lending Groups [Internet]. 2014. Available from: http://data .worldbank.org/about/country-and-lending-groups.
5. Commission on Macroeconomics and Health. Macroeconomics and Health: Investing in Health for Economic Development. Geneva: World Health Organization; 2001.
6. Chisholm D, Abegunde D, Mendis S, World Health Organization. Scaling up action against noncommunicable diseases: how much will it cost?. Geneva, Switzerland: World Health Organization; 2011.
7. Anderson BO, Lipscomb J, Murillo RH, Thomas DB. Breast Cancer. In Disease Control Priorities: Volume 3, Cancer, edited by H. Gelband, P. Jha, R. Sankaranarayanan, and S. Horton. 3rd ed. Washington, DC: World Bank.
8. Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, et al. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet. 2013 Aug;382(9890):452–77.
9. Rao S Seshadri, P Jha, P Sati. Karnataka’s roadmap to improved health: cost effective solutions to address priority diseases, reduce poverty and increase economic growth. Bangalore: Azim Premji University; 2015.
10. Organisation for Economic Co-Operation and Development (OECD). Cancer Care: Assuring Quality to Improve Survival. Paris: OECD Publishing; 2013.
11. Goss PE, Lee BL, Badovinac-Crnjevic T, Strasser-Weippl K, Chavarri-Guerra Y, Louis JS, et al. Planning cancer control in Latin America and the Caribbean. Lancet Oncol. 2013 Apr;14(5):391–436.
12. World Cancer Report 2014. Geneva: IARC; 2014.
13 Knaul FM, González-Pier E, Gómez-Dantés O, García-Junco D, Arreola-Ornelas H, Barraza-Lloréns M, Sandoval R, et al. The quest for universal health coverage: achieving social protection for all in Mexico. Salud Publica Mex. 2013;55(2):207–35.
14. Jamison DT, Summers LH, Alleyne G, Arrow KJ, Berkley S, Binagwaho A, et al. Global health 2035: a world converging within a generation. Lancet. 2013 Dec 7;382(9908):1898–955.
15. Knaul F, Horton S, Yerramilli P, Gelband H, Atun R. Financing Cancer Care in Low-Resource Settings. In Disease Control Priorities: Volume 3, Cancer, edited by H. Gelband, P. Jha, R. Sankaranarayanan, and S. Horton. 3rd ed. Washington, DC: World Bank.
16. Shisana O, Rehle T, Louw J, Zungu-Dirwayi N, Dana P, Rispel L. Public perceptions on national health insurance: moving towards universal health coverage in South Africa. South Afr Med J Suid-Afr Tydskr Vir Geneeskd. 2006 Sep;96(9):814–8.
17. Jha P, Laxminarayan R. Choosing health: an entitlement for all Indians. Toronto: Centre for Global Health Research; 2008.
18. Piot P, Quinn TC. Response to the AIDS pandemic–a global health model. N Engl J Med. 2013 Jun 6;368(23):2210–8.
19. Jamison DT, Mosley WH, Measham AR, Bobadilla JL. Disease Control Priorities in Developing Countries. Washington, D.C.: The World Bank; 1993.
20. Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, et al. Disease Control Priorities in Developing Countries, 2nd edition. Washington, DC: Oxford University Press and World Bank; 2006.
21. Dare AJ, Anderson BO, Sullivan R, Pramesh CS, Yip C-H, Ilbawi A, et al. Surgical services for cancer care. In Disease Control Priorities: Volume 3, Cancer, edited by H. Gelband, P. Jha, R. Sankaranarayanan, and S. Horton. 3rd ed. Washington, DC: World Bank.
22. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Peto R, Davies C, Godwin J, Gray R, Pan HC, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012 Feb 4;379(9814):432–44.